Expand your business in the U.S. with
From facility registration to product listing, labeling, and 24/7 expert support – we’ve got everything covered to ensure your seamless entry and growth in the U.S. market.
Get Your Free Consultation Today!

What is MoCRA?
FDA MoCRA, also known as the “Modernization of Cosmetics Regulation Act,” is a law enacted by the U.S. FDA to ensure the safety and efficacy of cosmetic products.
In response to the evolving market demands and the development of the 21st-century cosmetics industry, the FDA modernized its existing cosmetic regulations in 2022 through MoCRA. This act establishes requirements for cosmetic product registration, labeling, and safety assessments with the primary goal of protecting consumers.
Why does it matter?
All products categorized as cosmetics fall under the scope of FDA MoCRA registration.
According to The Federal Food, Drug, and Cosmetic Act (FFDCA), cosmetics are defined as products applied to the human body for purposes of cleansing, beautifying, enhancing attractiveness, or altering appearance.
Manufacturers and sellers of cosmetics must register all their products with the FDA when exporting to the United States.
Explore Our Services
Why Choose FDC Corp for Your U.S. Market Compliance?
Registration & US Agent
We handle everything from facility registration to product listing, labeling, and year-round support.
Product Listings
Eliminate customs and regulatory risks in advance, preventing recalls and market withdrawal.
Label and Ingredient Review
U.S. agents, real-time updates, and crisis management support tailored for your needs.
Compliance Training
Enhance your team’s capabilities and minimize internal risks.
HSA/FSA Market Entry
Secure HSA eligibility to target new sales channels and consumer segments.
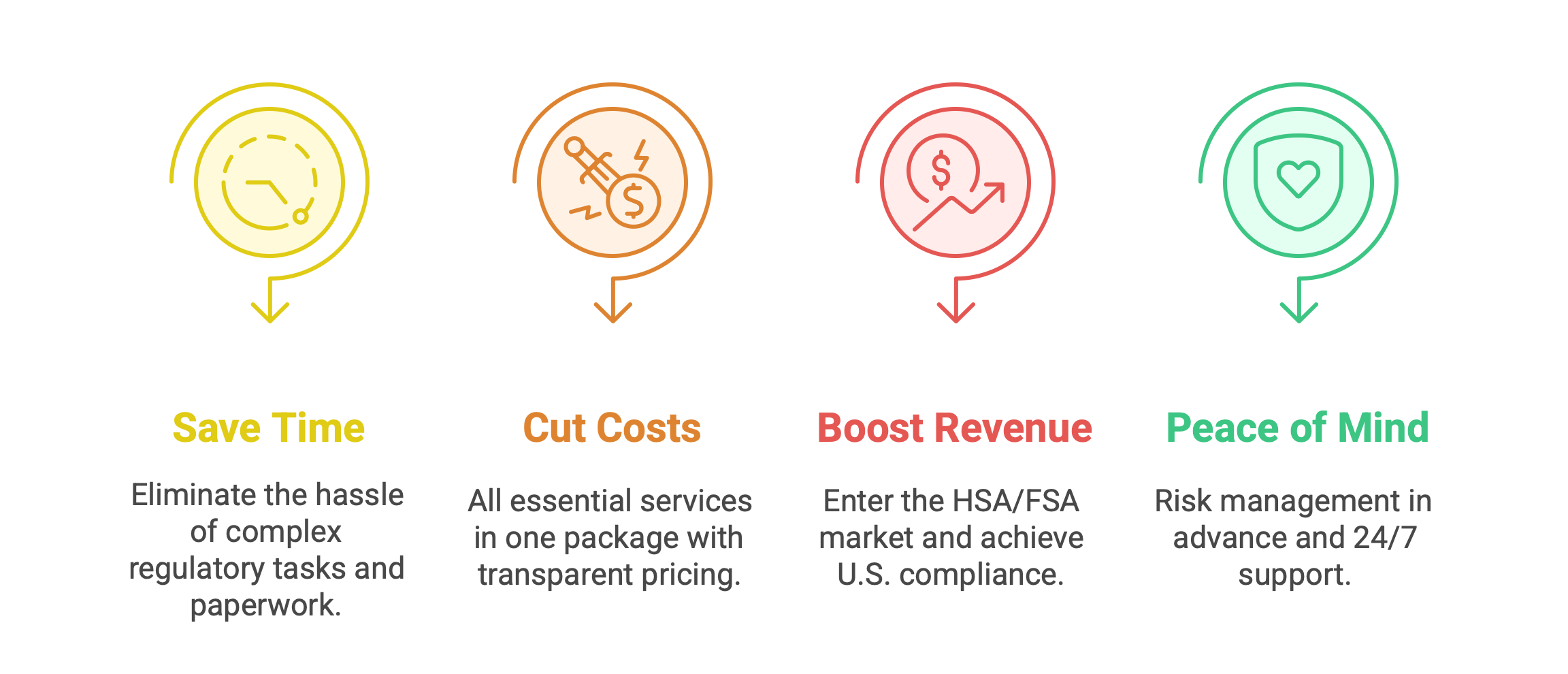
Save Time, Cut Costs, and Boost Revenue with FDC Corp
- Eliminate the hassle of complex regulatory tasks and paperwork.
- All essential services in one package with transparent pricing.
- Enter the HSA/FSA market + achieve complete U.S. compliance = expanded sales opportunities.
- Risk management in advance and 24/7 support to ensure your success.
Stay Updated with FDA Regulations
Latest Updates
Modernization of Cosmetics Regulation Act 2022
Stay informed about the latest changes and updates to the Modernization of Cosmetics Regulation Act.
Getting Started with MoCRA
Introduction: A New Era in Cosmetic Regulations U.S. beauty industry: the Modernization of Cosmetics
Get in Touch
Contact Us
Compliance for the U.S. Market + Revenue Growth. FDC Corp’s One-Stop Solution is here to help you.
Apply for Your Free Consultation Now!
Got any questions?
Contact us today
Stay Updated with FDA Regulations
The Responsible Person (RP) ensures product safety, maintains safety substantiation records, reports serious adverse events, and complies with labeling requirements. The RP must reside in the U.S. or have a physical business presence there. FDC Corp. can serve as your RP, ensuring compliance and seamless communication with the FDA.
MoCRA requires businesses to maintain:
- Safety substantiation records proving product safety.
- Adverse event reports for issues related to product use.
- Facility registration and product listing information, including detailed ingredient data.
- Labeling documentation, ensuring compliance with updated FDA rules.
FDC Corp. can assist in preparing and organizing these documents to ensure full compliance with MoCRA regulations.
Under MoCRA, cosmetic companies must:
- Register their manufacturing facilities with the FDA.
- Submit product listings, including detailed information on ingredients.
- Report serious adverse events to the FDA within 15 days.
- Ensure products are manufactured under GMPs.
- Comply with updated labeling requirements, including allergen disclosures.
Facilities manufacturing cosmetics for U.S. distribution must register and submit product listings unless exempt. Registration involves providing the facility’s FEI number, while product listing requires detailed ingredient information. Small businesses may have exemptions, but safety substantiation and adverse event reporting are still mandatory.
MoCRA applies to all cosmetic companies, regardless of size. Small businesses must comply with facility registration, product listing, and safety requirements. The FDA may offer tailored guidance or exemptions to reduce compliance burdens for small companies, but it’s essential to seek professional assistance to navigate these regulations effectively.

